Nanocoating specialists have made a breakthrough in combating nerve agents by developing an improved defensive textile for use in chemical weapons protective suits.
The nanotechnology is based on metal-organic frameworks (MOFs), a periodic nanoporous crystalline structure just a few nanometres thick with the ability to degrade toxic agents. The study claims that this atomically thin layer will work faster than current chemical protective materials which are based on blends of metal oxides and activated carbon.
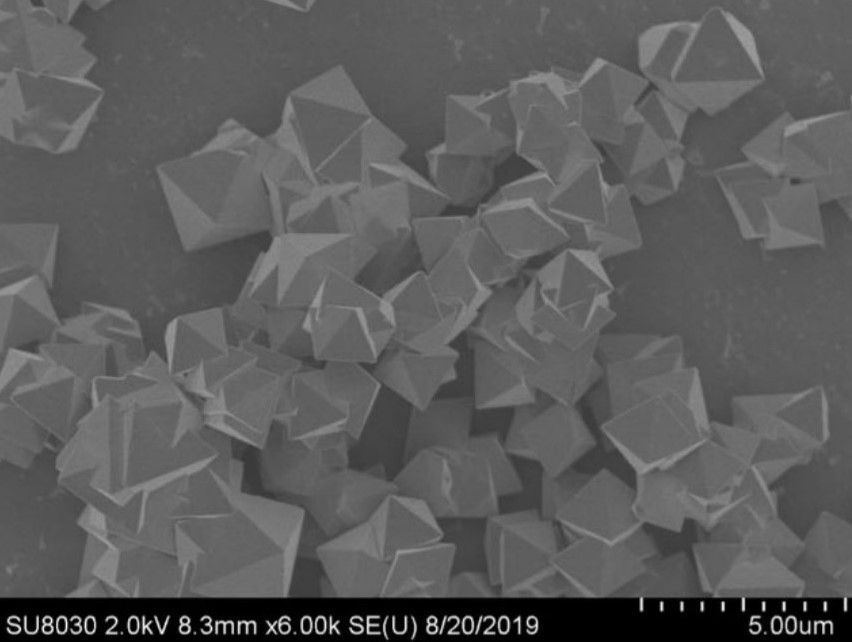
The MOFs are based on zirconium and work as a catalyst against chemical warfare agents, such as soman (GD) and VX. While previous studies had noted the ability of these nanocoatings to work in face masks and protective suits, they had always concluded that liquid water would be required for the nanotechnology to work effectively.
However, this latest research, from Northwestern University in America, found that the nanocoating could operate using only ambient humidity. This has led to the development of MOFs in chemical weapons suits, a novel application for a nanocoating which, due to its high surface area, is typically used for gas separation, in sensing devices, or chemical storage.
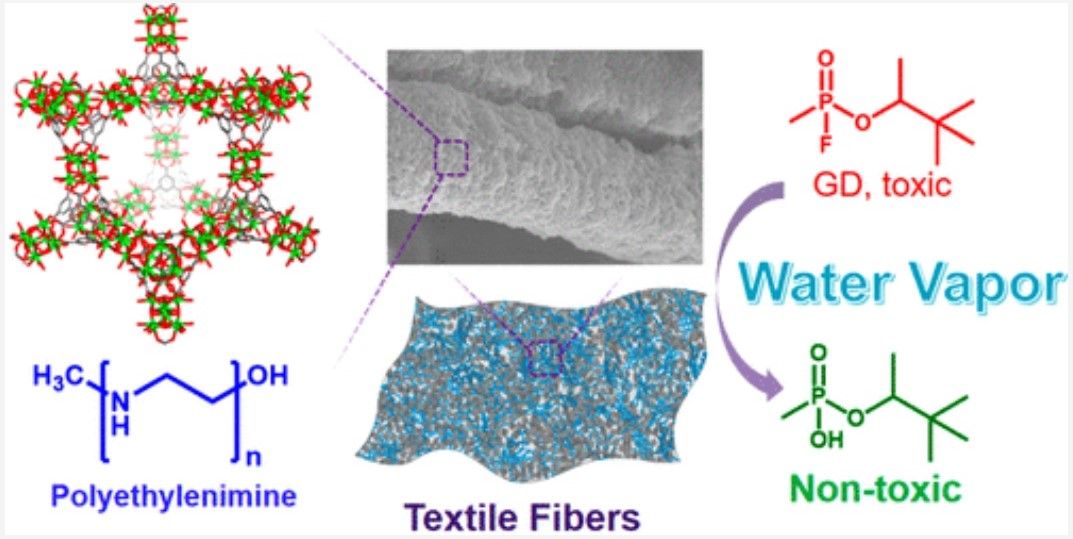
The study was founded on organising the frameworks metal cluster around organic ligands in a way that allowed the chemical warfare agents to be captured inside the nanopores. Once there, the nanocoating can degrade them in a catalytic hydrolysis reaction until they are safe.

The technical aspects of the study were reported in the scientific journal Physics World, which describes how, “In their experiments, the team studied a Zr-based MOF with the chemical formula [Zr6O4(μ3-OH)4(OH)6(H2O)6(BTC)2]·nH2O (more commonly known as MOF-808). They began by applying a solution containing this material and polyethylenimine (PEI) to strips of cotton fabric. After allowing the fabric to dry overnight, they exposed it to DMNP, a nerve agent simulant commonly used in academic research labs. These tests showed that the MOF/PEI composite degraded DMNP even in the absence of water.”

The researchers, led by Omar Farha, an associate professor of chemistry at Northwestern, have now published their findings in the Journal of the American Chemical Society (JACS) where they explain how, “Metal–organic frameworks (MOFs) are promising candidates for the catalytic hydrolysis of nerve agents and their simulants.”
The report continues by outlining how the breakthrough is, “… a generalizable and scalable approach for integrating MOFs and non-volatile polymeric bases onto textile fibers for nerve agent hydrolysis…which can significantly reduce the dimensions of filters and increase the efficiency of protective suits.”
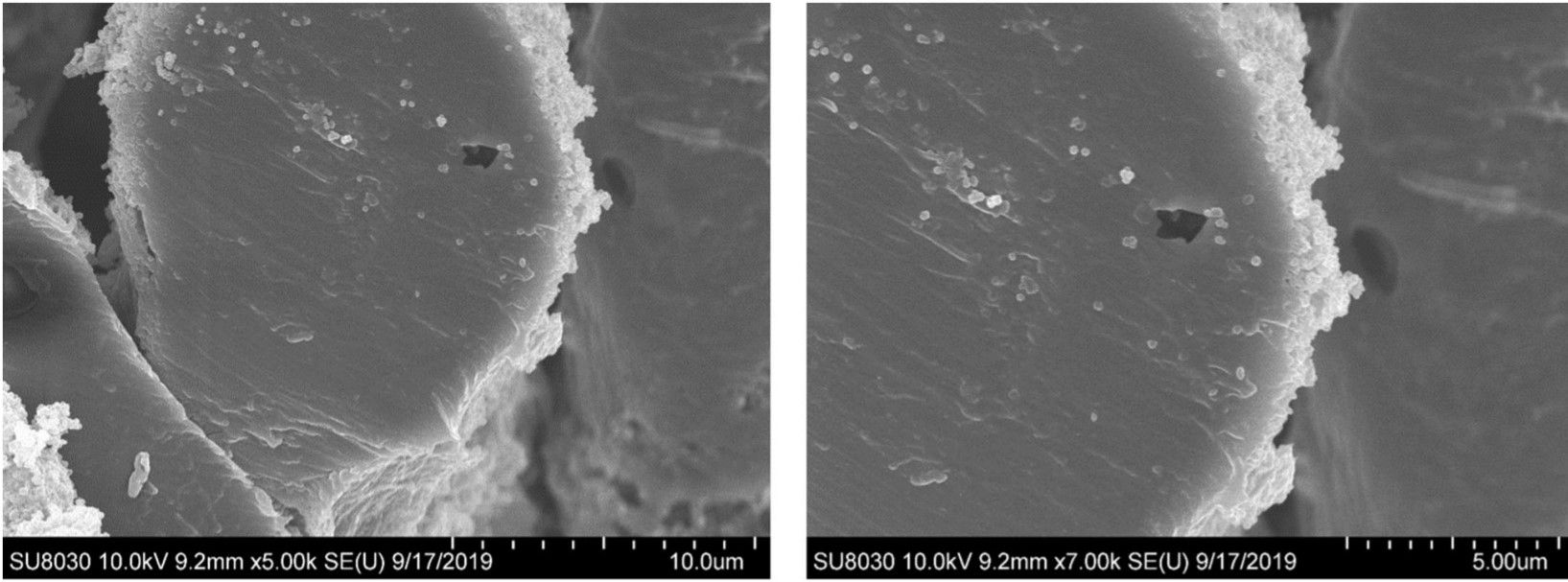
The team have now tested the material’s durability to see if it is a practical coating for use in a battlefield scenario.
These experiments found that the MOF remained securely attached to the cotton and maintains its crystalline framework even when immersed in water and agitated for 24 hours. Catalytic activity also remained high after exposure to ambient air for 100 days.

Further tests exposed the treated cotton to ‘real world’ chemicals such as atmospheric carbon dioxide and pollutants, such as octane. The material was even exposed to sweat, all without limiting the effectiveness of the nanocoating.
The team have now set themselves further goals, planning to develop nanocoatings for protective suits which can detoxify chemical weapons even more quickly, or that can handle numerous different poisons simultaneously.
As Farha notes, “We are interested in designing fabrics that can degrade multiple agents at the same time.”
The success or failure of the team and their nanocoatings could lead to a safer world and help to make chemical weapons a far less ominous threat than they are today.
