So much of the chemical industry is based on the power of catalysts. They make the chemical processes upon which our daily lives depend and drive the industry forward. When it comes to making chemical products, catalysts are everywhere.
And correspondingly, billions of dollars of chemical industry investment is based upon the development of newer and better catalysts.
Now, nanotechnology has taken a great step forward into aiding the chemical industry with the creation of a nanoscale lattice of palladium and yttrium for a more efficient and sustainable catalyst.
The study was conducted at the Tokyo Institute of Technology and is based on a palladium-based intermetallic electride called Y3Pd2 which has been shown to improve the efficiency of carbon-carbon cross-coupling reactions.
Such is the potential for this nanomaterial solution to find a more sustainable route to catalysis for the chemical industry that the study has now been published in the journal Nature Communications, rather than a specialist nanotechnology review journal.
In the journal they state that, “The results confirmed the general applicability of the Y3Pd2 catalyst with high chemoselectivity and functional group tolerance.”
In simpler terms, the team have developed an electride material that can be used for Suzuki cross-coupling reactions to form carbon-carbon bonds in organic and medicinal chemistry. The production of these reactions are some of the most commonly used processes in the chemical industry.
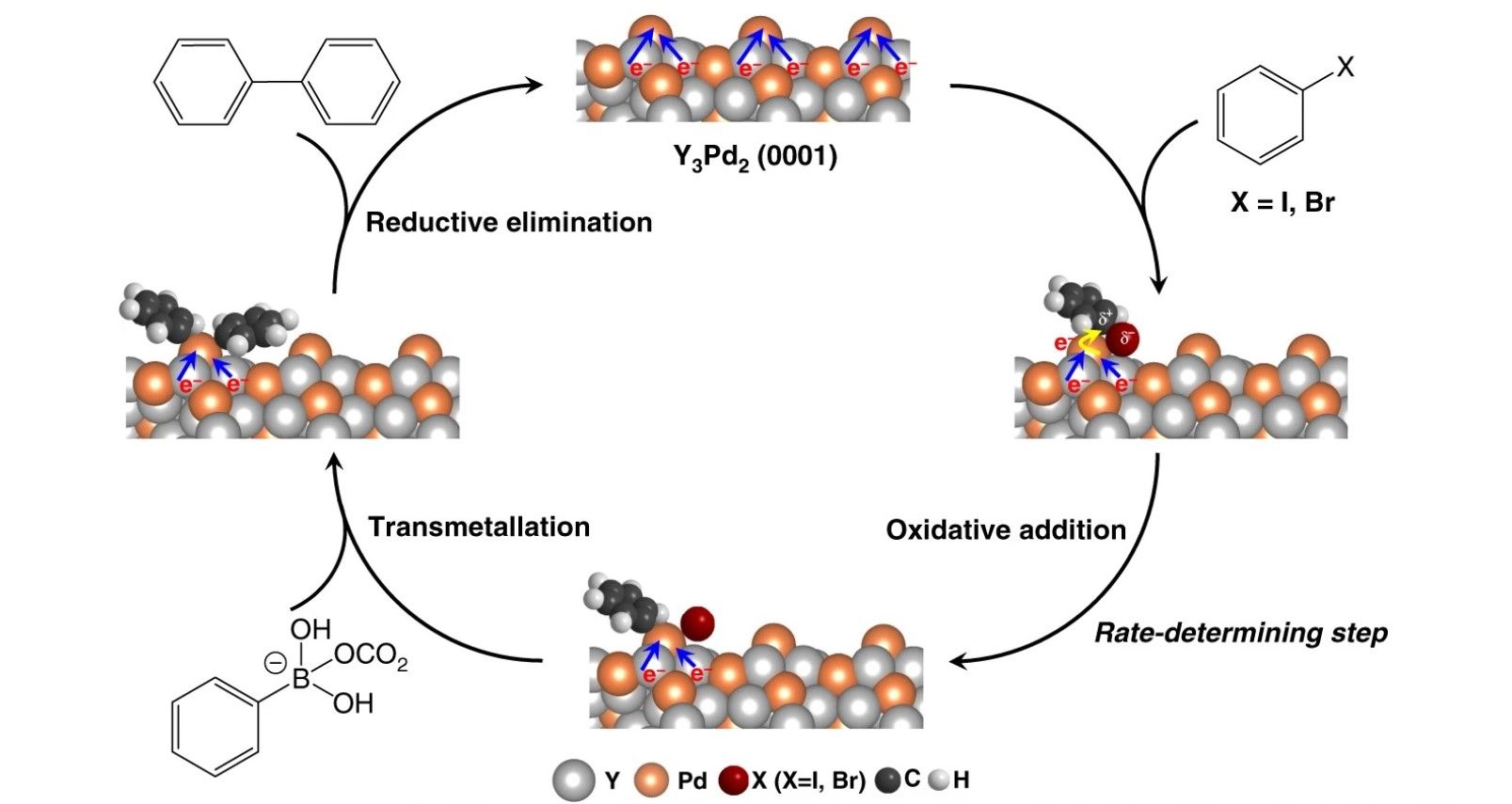
“In an electride,” explains Tian-Nan Ye, an assistant professor at Tokyo Tech's Materials Research Center for Element Strategy and the study’s first author, “anionic electrons are trapped in interstitial sites and typically host a strong electron donation effect. This feature motivated us to apply Y3Pd2 as a Suzuki coupling reaction catalyst, as the reaction barrier of the rate-determining step can be suppressed through electron transfer from the electride to the substrates.”
Ultimately, tests have shown that the new catalyst is 10 times more active than pure Pd catalysts, while the activation energy was 35% less. The newfound efficiency is due to the active Pd atoms held inside the nanoscale lattice.
In addition, the new catalyst is highly reusable, remaining effective for as many as 20 cycles, while the Pd atoms can also be recovered relatively easily. This makes the process more sustainable and offers an increase in efficiency that would represent major economic savings across the industry.
“The stabilized Pd active sites in our crystalline lattice solve the problems of aggregation and leaching that have commonly occurred in other systems reported so far,” says Ye. “This makes our catalyst extremely robust and stable for long-term usage, without deactivation.”
However, the original idea of combining yttrium and palladium was made by Jens Kehlet Nørskov, a researcher now based at Stanford University.
As the Tokyo Institute of Technology’s press release reports, “In 2009, Nørskov and co-workers published ground-breaking findings on catalysts made of platinum alloyed with early transition metals, including yttrium. Since then, many groups have been investigating new combinations of intermetallic compounds (consisting of a rare earth metal and an active transition metal), with the goal of developing much more efficient catalysts for the chemical industry.”
Adding that, “Through a series of calculations and experimental studies, Ye and his team showed that Y3Pd2 has a strong electron-donating effect associated with a low work function and high carrier density -- features that enable the catalyst to work at a much lower activation energy than that of a pure Pd catalyst.”
The researchers are now continuing their studies to try and resolve the problem of the new catalyst’s relatively low surface area. At present, this involves experimenting with a pulverizing technique called ball-milling, as well as examining the effects of solvents, such as ethanol and heptane.
To date, this analysis has found that the larger the surface area the larger the rate of catalysis.
However, regardless of the outcome of this further work, the team are satisfied that nanomaterials are part of a significant breakthrough for the production of a wide range of chemical products. A route to making more sustainable chemicals more efficiently.
Photo credit: Nature, Springer, Titech, nonrepititous66 & Azno
